Simply Sublime Phase Change Lab Digital learning, Chemistry

This lab is a simple activity that has students investigate the process of sublimation. I use this lab after teaching about heating curves and phase diagrams. It allow's them to reflect on the difference between the heating curve of water and what occurs during the process of sublimation. The file i
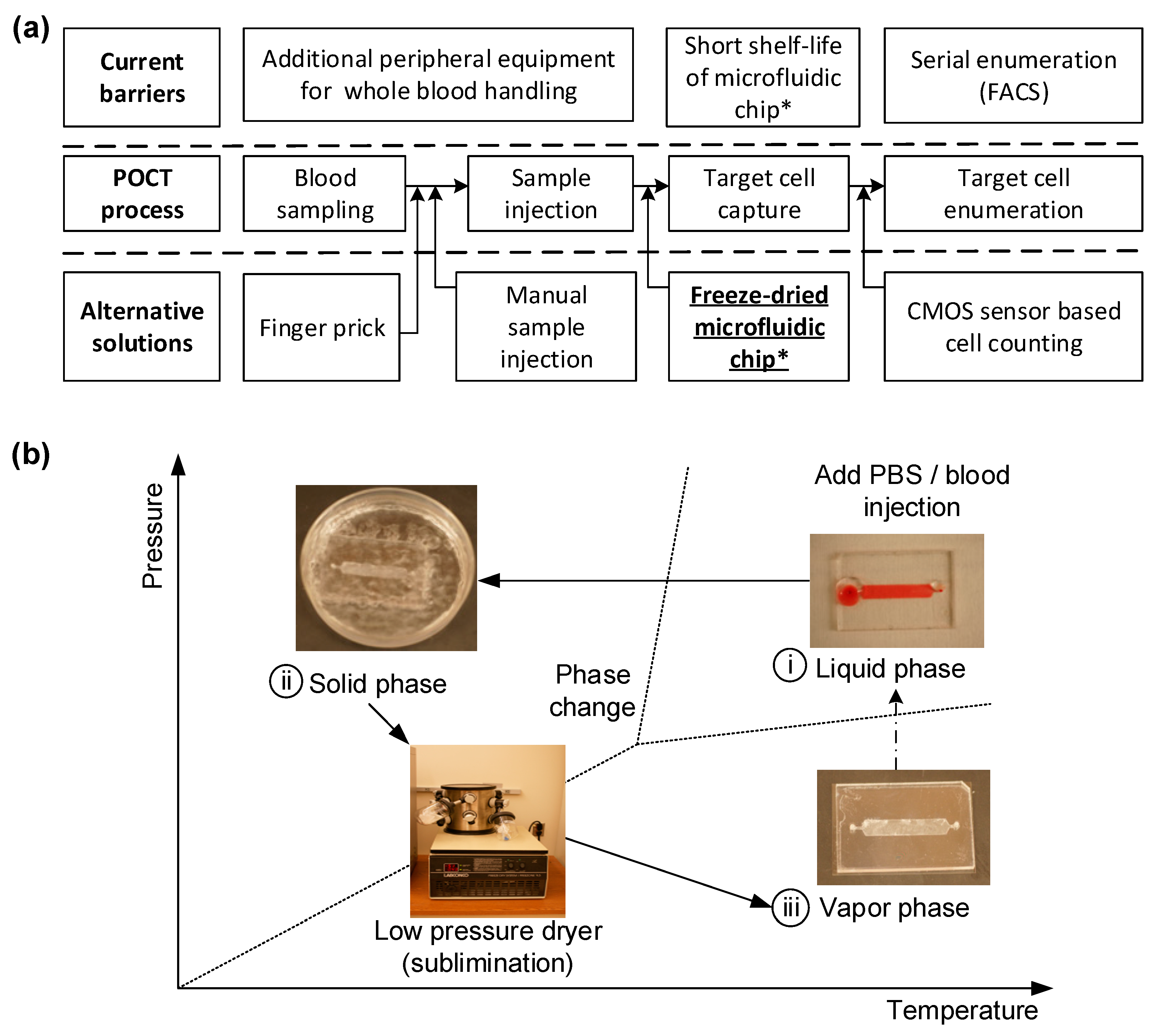
Sensors, Free Full-Text
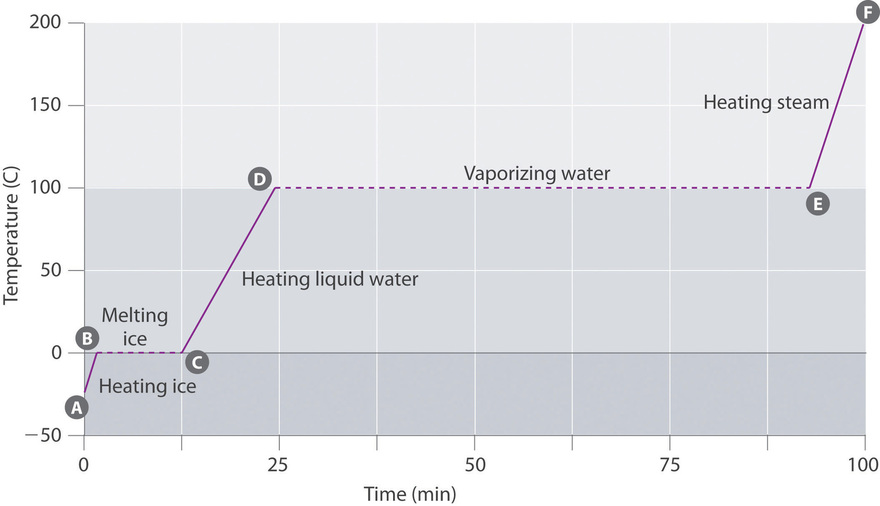
3.2: Energy of Phase Changes - Chemistry LibreTexts
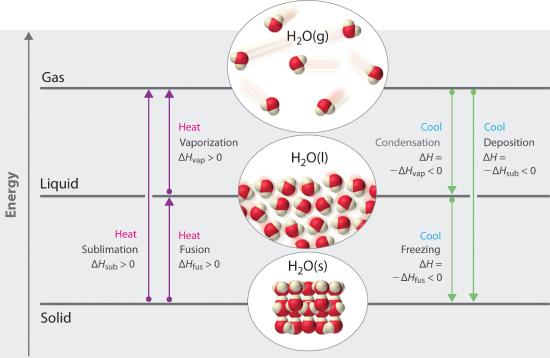
Chapter 11.5: Changes of State - Chemistry LibreTexts

10.3 Phase Change Diagrams

The Ongoing Mystery of Covid's Origin - The New York Times

Kami Export - Copy of Phase change interactive lab - IS2-1 lesson 1- PhET phase change interactive - Studocu

SuperSimple Chemistry: The Ultimate Bitesize Study Guide [1 ed.] 1465493239, 9781465493231

Sublimation - Also, to become familiar with the phase changes which require you to characterize the - Studocu

Sublimation - Also, to become familiar with the phase changes which require you to characterize the - Studocu
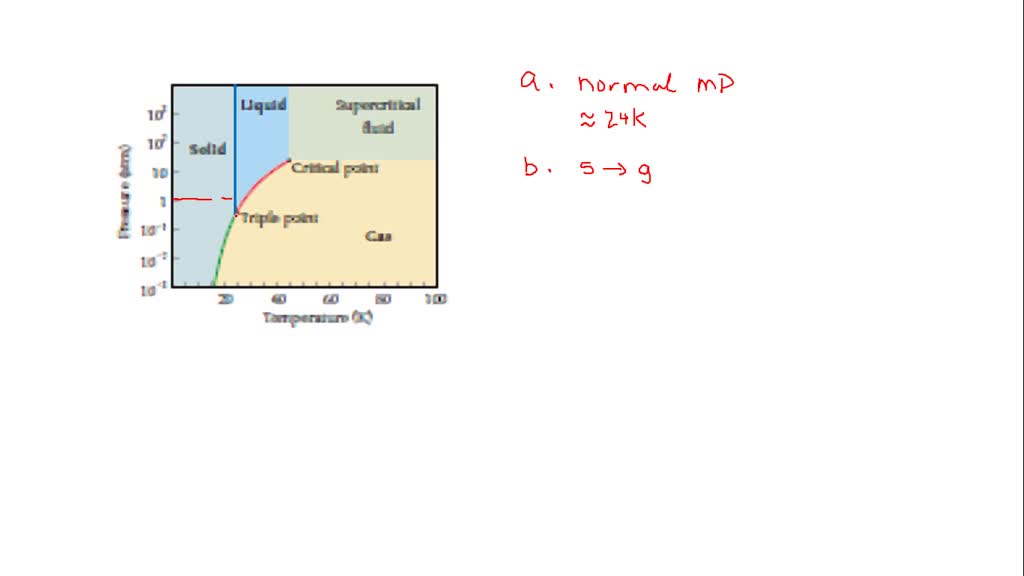
⏩SOLVED:The phase diagram for neon is Use the phase diagram to…

Calaméo - IASP 2023 - Conference Proceedings
An engaging lab activity designed to enhance students’ understanding of how a substance’s state of matter changes when thermal energy is added or

5-PS1-2 Phase Change Lab
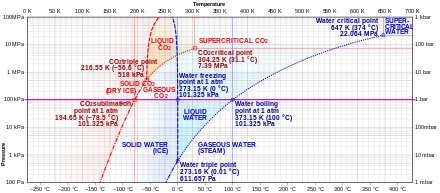
Sublimation (phase transition) - Wikipedia
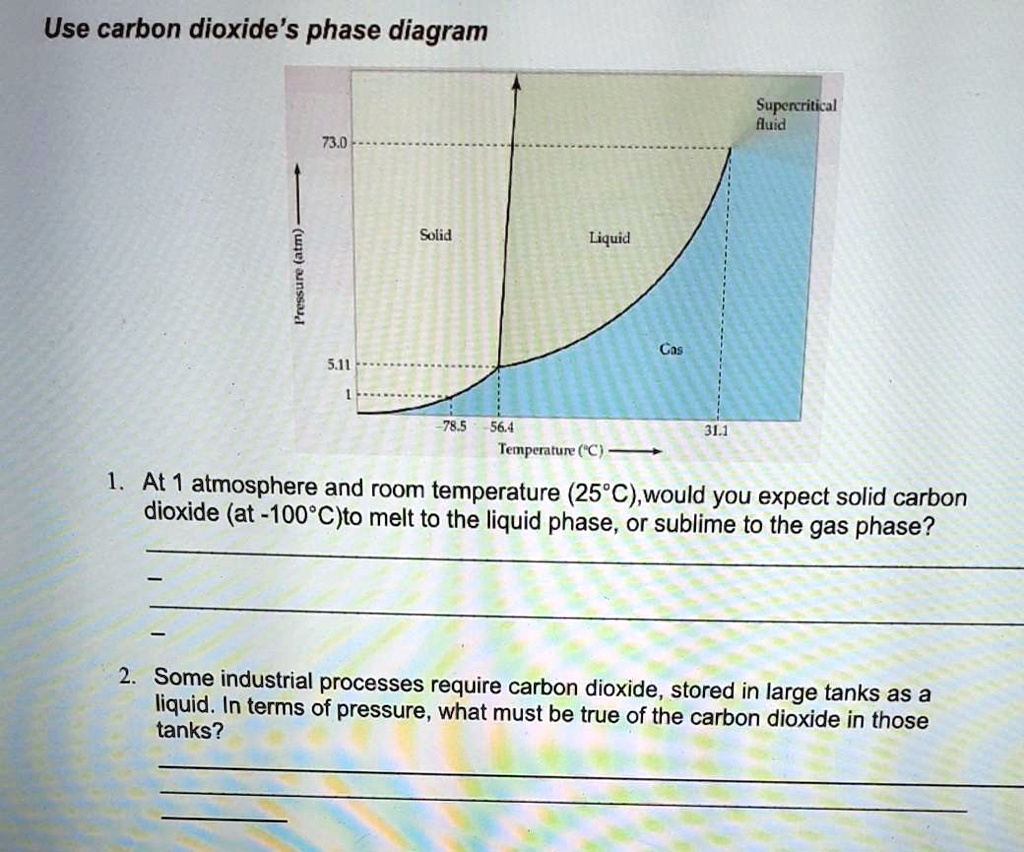
SOLVED: Use carbon dioxide's phase diagram Supercritical fluid 71.0 Solid Liquid Gas 56.4 Temperature (°C) At 1 atmosphere and room temperature (25°C), would you expect solid carbon dioxide (at -100°C) to melt

Predict whether or not the substances in the table will sublime at STP. Base your predictions only on the







